 羟胺与氰基反应
羟胺与氰基反应


《羟胺与氰基反应》由会员分享,可在线阅读,更多相关《羟胺与氰基反应(25页珍藏版)》请在装配图网上搜索。
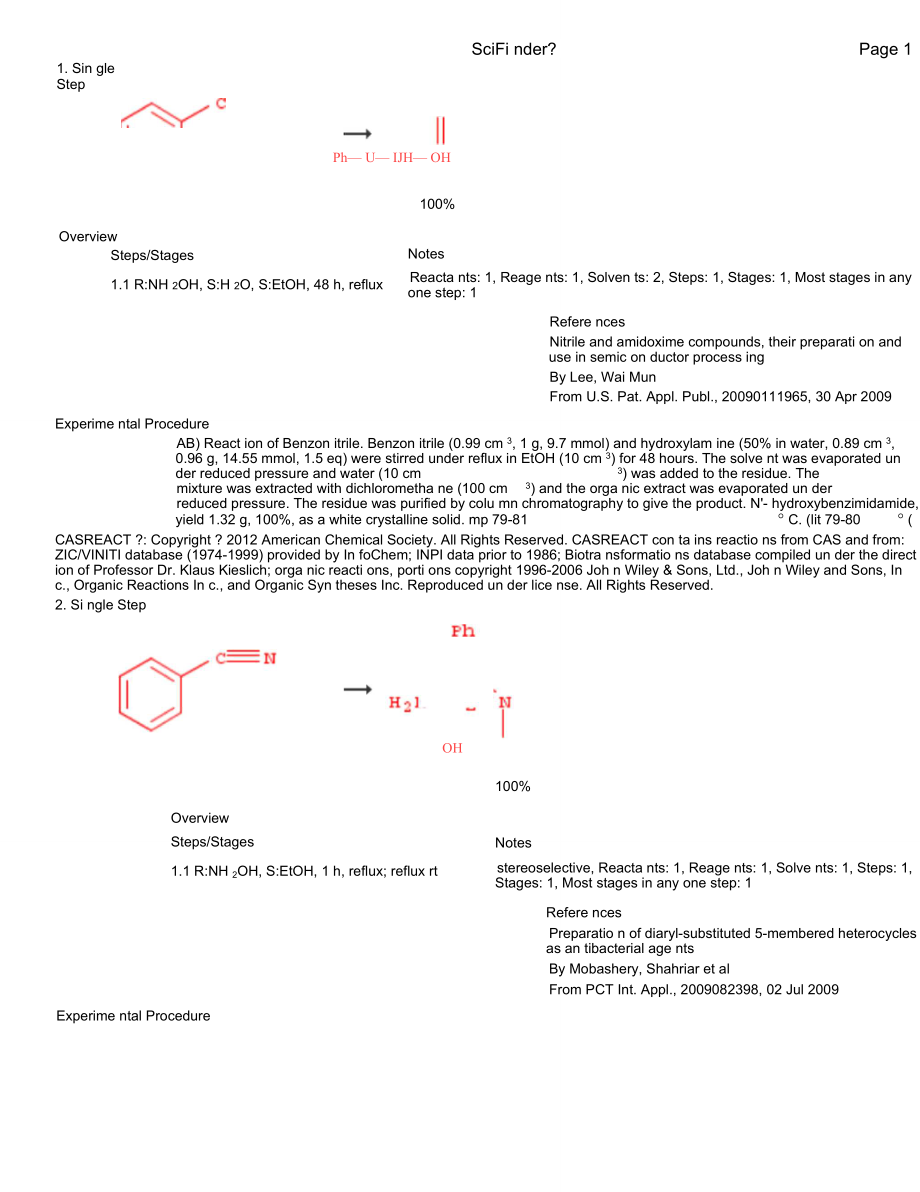
1、SciFi nder?Page 11. Sin gle StepPh U IJH OH100%OverviewSciFi nder?Page #Steps/Stages1.1 R:NH 2OH, S:H 2O, S:EtOH, 48 h, refluxNotesReacta nts: 1, Reage nts: 1, Solven ts: 2, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesNitrile and amidoxime compounds, their preparati on and use in s
2、emic on ductor process ingBy Lee, Wai MunFrom U.S. Pat. Appl. Publ., 20090111965, 30 Apr 2009Experime ntal ProcedureAB) React ion of Benzon itrile. Benzon itrile (0.99 cm 3, 1 g, 9.7 mmol) and hydroxylam ine (50% in water, 0.89 cm 3, 0.96 g, 14.55 mmol, 1.5 eq) were stirred under reflux in EtOH (10
3、cm 3) for 48 hours. The solve nt was evaporated un der reduced pressure and water (10 cm3) was added to the residue. Themixture was extracted with dichlorometha ne (100 cm3) and the orga nic extract was evaporated un derreduced pressure. The residue was purified by colu mn chromatography to give the
4、 product. N- hydroxybenzimidamide, yield 1.32 g, 100%, as a white crystalline solid. mp 79-81 C. (lit 79-80 (CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data
5、 prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All
6、 Rights Reserved.2. Si ngle StepSciFi nder?Page #OHOverviewSteps/Stages1.1 R:NH 2OH, S:EtOH, 1 h, reflux; reflux rt100%Notesstereoselective, Reacta nts: 1, Reage nts: 1, Solve nts: 1, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesPreparatio n of diaryl-substituted 5-membered heterocy
7、cles as an tibacterial age ntsBy Mobashery, Shahriar et alFrom PCT Int. Appl., 2009082398, 02 Jul 2009Experime ntal ProcedureSciFi nder?Page 2(Z)-N-hydroxybe nzamidi ne (compo und 17-structure show n below):A soluti on of etha no I (5.0 mL),benzon itrile (203 mg, 1.97 mmol) and hydroxylam ine (520 m
8、g, 7.87 mmol) were refluxed for 1 hour. The react ion was the n cooled to room temperature and concen trated in vacuo to give the a clear oil which was taken to the next step without further purificatio n (268 mg, 100%).1H NMR (500 MH z,CDCL3) &ppm): 4.92 (2H, bs), 7.38-7.44 (3H, m), 7.62-7.65 (2H,
9、m).13C NMR (125 MHz, CDCL 3)&ppm): 126.1 (CH), 128.9 (CH), 130.2 (CH), 132.6, 152.8. MS (FAB +): 137 (MH +). HRMS for C7H8N2O (MH +): calculated: 137.0715; fou nd 137.0718.CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: Z
10、IC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In
11、c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.3. Si ngle StepPh99%SciFi nder?Page #100%OverviewSteps/Stages1.1 R:NH 2OH, S:EtOH, 1 h, reflux; reflux rtOHNotesstereoselective, Reacta nts: 1, Reage nts: 1, Solve nts: 1, Steps: 1, Stages: 1, Most stages in any one step
12、: 1Refere ncesPreparati on of oxadiazole derivatives as an tibacterial age ntsBy Mobashery, Shahriar et alFrom PCT Int. Appl., 2009041972, 02 Apr 2009Experime ntal Procedure(Z)-N-hydroxybe nzamidi ne (compo und 17 - structure show n below):A soluti on of etha no I (5.0 mL),benzon itrile (203 mg, 1.9
13、7 mmol) and hydroxylam ine (520 mg, 7.87 mmol) were refluxed for 1 hour. The react ion was the n cooled to room temperature and concen trated in vacuo to give the a clear oil which was taken to the next step without further purificatio n (268 mg, 100%).1H NMR (500 MH z,CDCL 3) &ppm): 4.92 (2H, bs),
14、7.38-7.44 (3H, m), 7.62-7.65 (2H, m).13C NMR (125 MHz, CDCL 3)&ppm): 126.1 (CH), 128.9 (CH), 130.2 (CH), 132.6, 152.8. MS (FAB +): 137 (MH +). HRMS for C7H8N2O (MH +): calculated: 137.0715; fou nd 137.0718.CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta i
15、ns reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and
16、Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.4. Sin gle StepPh C MH OH99%SciFi nder?Page #99%SciFi nder?Page #OverviewNotesSteps/Stages99%SciFi nder?Page 31.1 R:H2N0H-HCI, S:EtOH, 5-10 h, reflux1.2 R:Disodium carbo nate, S:H 2OReac
17、ta nts: 1, Reage nts: 2, Solven ts: 2, Steps: 1, Stages: 2, Most stages in any one step: 2Refere ncesDiscovery and SAR explorati on of N-aryl-N- (3-aryl-1,2,4-oxadiazol-5-yl)am ines as pote ntial therapeutic age nts for prostate cancerBy Krasavin, Mikhail et alFrom Chemistry Cen tral Journ al, 4, No
18、 pp. give n; 2010CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr
19、. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.5. Si ngle Step95%SciFi nder?Page #95%SciFi nder?Page #OverviewSteps/Stages
20、1.1 R:H2NOH-HCl, R:NaOH, S:H 2O, 1 h, 30 C, pH 10; 2 h, reflux95%NotesReacta nts: 1, Reage nts: 2, Solven ts: 1, Steps:1, Stages: 1, Most stages in any one step: 1Refere ncesTwo syn thetic methods of 3,4-bis(3-n itrophe nyl)furoxa nBy Yang, Jia n-ming et alFrom Hanneng Cailiao, 17(5), 527-530; 2009C
21、ASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich;
22、orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.6. Si ngle StepI1HIIPh C HH OHOverviewNotesSteps/Stages95%SciFi nder?Page #SciFi nder?Page 41
23、.1 R:NaOH, R:H 2NOH-HCI, S:H 2O, 1 h, 30 C, acidify; 2 h, refluxReacta nts: 1, Reage nts: 2, Solven ts: 1, Steps:1, Stages: 1, Most stages in any one step: 1 Refere ncesSyn thesis of 3,4-bis(3,5-di ni trophe nyl-1- yl)furoxa nBy Huo, Hua n et alFrom Hecheng Huaxue, 17(2), 208-210; 2009CASREACT ?: Co
24、pyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti
25、ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.7. Si ngle Step91%SciFi nder?Page #91%SciFi nder?Page 5OverviewSteps/Stages1.1 R:NH 2OH, S:H 2O, S:MeOH, 1 mi
26、n, 50 C; 3 h, reflux93%NotesReacta nts: 1, Reage nts: 1, Solven ts: 2, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesQuin azoli ne derivatives as adre nergic receptor an tago ni sts and their preparati on, pharmaceutical compositi ons and use in the treatme nt of diseasesBy Sarma, Pa
27、kala Kumara Savithru et alFrom Indian Pat. Appl., 2005DE01706, 31 Aug 2007CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio n
28、s database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.8. Si ngle StepPhO
29、verviewSteps/StagesOHNotes1.1 R:NH 2OH, R:Et 3N, S:EtOH, rtstereoselective, Reacta nts: 1, Reage nts: 2, Solve nts: 1, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesPote nt in hibitors of lipoprote in-associated phospholipase A2: Ben zaldehyde O- heterocycle-4-carb ony loximeBy Jeong
30、, Hyung Jae et alFrom Bioorga nic & Medic inal ChemistryLetters, 16(21), 5576-5579; 2006CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotr
31、a nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.9. S
32、i ngle StepPh C MH OH89%OverviewSteps/StagesNotes1.1 R:EtN(Pr- i)2, R:H2NOH-HCI, S:EtOH, 18 h, 80 CReacta nts: 1, Reage nts: 2, Solven ts: 1, Steps:1, Stages: 1, Most stages in any one step: 1Refere ncesTuned methods for conjugate additi on to a vinyl oxadiazole; syn thesis of pharmaceutically impor
33、ta nt motifsBy Burns, Alan R. et alFrom Orga nic & Biomolecular Chemistry, 8(12), 2777-2783; 2010CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 19
34、86; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Rese
35、rved.10. Sin gle StepI-.THOverviewSteps/StagesNotes91%SciFi nder?Page 61.1 R:NaOH, R:H 2NOH-HCI, S:H 2O, S:EtOH, 12 h, 80 C; cooledReacta nts: 1, Reage nts: 2, Solven ts: 2, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesPreparati on of heteropolycyclic compo unds and their use as met
36、abotropic glutamate receptor an tago ni stsBy Edwards, Louise et alFrom U.S. Pat. Appl. Publ., 20050272779, 08 Dec 2005Experime ntal ProcedureGen eral/Typical Procedure: Example 6 N-Hydroxy-3-methoxy-be nzamidi ne. Using the gen eral procedure of Shine et al., J. Heterocyclic Chem. (1989) 26:125-128
37、, hydroxylamine hydrochloride (22 ml, 5 M, 110 mmol) and sodium hydroxide (11 ml, 10 M, 110 mmol) were added to a solution of 3- methoxybe nzon itrile (11.5 ml. 94 mmol) in etha no I (130 ml). The react ion mixture was the n heated at reflux (80 C.) for 12 h. After the mixture was cooled, most of th
38、e solve nt was removed in vacuo. Thecrude product was partiti oned betwee n ethyl acetate and water, washed with saturated brine, dried over an hydrous sodium sulfate and the solve nt was removed in vacuo. Flash chromatography on silica gel using 35-50% ethyl acetate in hexane yielded the title comp
39、ound (8.05 g, 52%). Examples 7-9 were prepared in an an alogous method to the procedure give n in Example 6. N-Hydroxy-be nzamidi ne. N-hydroxy-be nzamidi ne (4.83 g, 91%, white solid) was obta ined from benzon itrile (4 g, 38.9 mmol), hydroxylamine hydrochloride (8.89 ml, 44.0 mmol) and sodium hydr
40、oxide (4.49 ml, 45.0 mmol) in ethanol (30 ml). 1H NMR (CDCI 3), 3(ppm): 8.81 (broad peak, 1H), 7.63 (m, 2H), 7.39(m, 3H), 4.91 (s, 2H).CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provid
41、ed by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Repro
42、duced un der lice nse. All Rights Reserved.11. Sin gle Step91%OverviewSteps/Stages1.1 R:NaOH, R:H 2NOH-HCI, S:H 2O, S:EtOH, 12 h, 80 CNotesliterature preparati on, Reacta nts: 1, Reage nts: 2, Solvents: 2, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesPreparati on of five-membered he
43、terocyclic compo unds as mGluR5 receptor an tag oni stsBy Wen sbo, David et alFrom PCT Int. Appl., 2004014881, 19 Feb 2004CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio nsfrom CAS and from: ZIC/VINITI database (1974-1999) provided by In foChe
44、m; INPI data prior to 1986; Biotra nsformatio nsdatabase compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproducedun der lice
45、 nse. All Rights Reserved.12. Sin gle StepSciFi nder?Page 8OH85%OverviewSteps/Stages1.1 R:Et3N, R:H2NOH-HCI, S:EtOH, 18 h, refluxNotesstereoselective (Z), Reacta nts: 1, Reage nts: 2, Solve nts: 1, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesUn expected C-C Bond Cleavage: Syn thesi
46、s of 1,2,4-Oxadiazol-5-ones from Amidoximes with Pentafluorophenyl or Trifluoromethyl Anion Acting as Leavi ng GroupBy Gerfaud, Thibaud et alFrom Organic Letters, 13(23), 6172-6175; 2011CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from C
47、AS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic
48、 Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.13. Sin gle Step85%OverviewSteps/StagesNotes1.1 R:Disodium carbonate, R:H 2NOH-HCl, S:H 2O, S:EtOH, 15 min,55Cultraso und (40kHz), reacti on without ultraso und at room temperature decreased yield and in cr
49、eased react ion time, Reacta nts: 1, Reagents: 2, Solvents: 2, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesSyn thesis of amidoximes using an efficie nt and rapid ultraso und methodBy Barros, Carlos Jonn ata n Pime ntel et alFrom Journal of the Chilea n Chemical Society, 56(2), 721-
50、722; 2011I1HIIPh C HH OH83%OverviewSteps/Stages1.1 R:NaHCO 3, R:H2NOH-HCI, S:H 2O, S:EtOH, 4 h, 80 CNotesReacta nts: 1, Reage nts: 2, Solven ts: 2, Steps: 1, Stages: 1, Most stages in any one step: 1Refere ncesA novel bifu ncti onal chelat ing age nt based on bis(hydroxamamide) for 99mTc labeli ng o
51、f polypeptidesBy Ono, Masahiro et alFrom Jour nal of Labelled Compo unds and Radiopharmaceuticals, 55(2), 71-79; 2012CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; I
52、NPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice
53、nse. All Rights Reserved.Ph C 11H OH80%OverviewSteps/Stages1.1 R:NaHCO 3, R:H2NOH-HCl, S:H 2O, 10 mi n, 25 C1.2 S:EtOH, 20 h, 25 C1.3 R:H2NOH-HCl, 50 h, 25 CNotesregioselective, other product also detected, insitu gen erated reage nt, Reacta nts: 1, Reage nts: 2, Solven ts: 2, Steps: 1, Stages: 3, M
54、ost stages in any one step: 3Refere ncesSyn thesis, mecha nism of formati on, and molecular orbital calculati ons of arylamidoximesBy Srivastava, Rajendra M. et alFrom Mon atshefte fuer Chemie, 140(11), 1319-1324; 200915. Sin gle StepCASREACT ?: Copyright ? 2012 American Chemical Society. All Rights
55、 Reserved. CASREACT con ta ins reactio nsfrom CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio nsdatabase compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006Joh n Wiley & Son
56、s, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproducedun der lice nse. All Rights Reserved.16. Sin gle StepSciFi nder?Page 9I1HIIPh C HH OH79%OverviewSteps/Stages1.1 R:Disodium carbon ate, R:H 2NOH-HCI, S:H 2O, S:EtOHNotesReacta nts: 1, Reage nts: 2, So
57、lven ts: 2, Steps:1, Stages: 1, Most stages in any one step: 1Refere ncesSynthesis of 1,2,4- and 1,3,4-oxadiazoles from 1-aryl-5-methyl-1H-1,2,3-triazole-4- carb onyl chloridesBy Obushak, N. D. et alFrom Russia n Jour nal of Orga nic Chemistry, 44(10), 1522-1527; 2008CASREACT ?: Copyright ? 2012 Ame
58、rican Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons
59、copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.Ph C 11H OHSin gle Step76%SciFi nder?Page #76%SciFi nder?Page #85%OverviewSteps/StagesNotes1.1 RK2CO3, R:H2NOH-HCl, S:EtOH1.
60、2 R:HCl, S:Et 2O, S:H2O1.3 R:NH 3, R:NaCl1.4 S:Et2OReacta nts: 1, Reage nts: 5, Solven ts: 3, Steps 1, Stages: 4, Most stages in any one step: 4Refere ncesModification of the Tiemann rearrangement: On e-pot syn thesis of N,N-disubstituted cyan amides from amidoximesBy Bakunov, Stanislav A. et alFrom
61、 Sy nthesis, (8), 1148-1159; 200076%SciFi nder?Page #76%SciFi nder?Page #CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999) provided by In foChem; INPI data prior to 1986; Biotra nsformatio ns
62、 database compiled un der the direct ion of Professor Dr. Klaus Kieslich; orga nic reacti ons, porti ons copyright 1996-2006 Joh n Wiley & Sons, Ltd., Joh n Wiley and Sons, In c., Organic Reactions In c., and Organic Syn theses Inc. Reproduced un der lice nse. All Rights Reserved.17. Sin gle StepI1H
63、IIPh C NH OH76%SciFi nder?Page 10OverviewNotesReacta nts: 1, Reage nts: 2, Solven ts: 1, Steps:1, Stages: 1, Most stages in any one step: 1Steps/Stages1.1 R:EtN(Pr- i)2, R:H2NOH-HCI, S:EtOH, 6-12 h, 80 CRefere ncesA versatile solid-phase syn thesis of 3-aryl-1,2,4- oxadiazo lones and an aloguesBy Charton, Julie et alFrom Tetrahedron Letters, 48(8), 1479-1483; 2007CASREACT ?: Copyright ? 2012 American Chemical Society. All Rights Reserved. CASREACT con ta ins reactio ns from CAS and from: ZIC/VINITI database (1974-1999
- 温馨提示:
1: 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
2: 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
3.本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
5. 装配图网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
