 生物化学英文课件:Chapter4 Proteins Three-Dimensional Structures and Function(part 2)
生物化学英文课件:Chapter4 Proteins Three-Dimensional Structures and Function(part 2)


《生物化学英文课件:Chapter4 Proteins Three-Dimensional Structures and Function(part 2)》由会员分享,可在线阅读,更多相关《生物化学英文课件:Chapter4 Proteins Three-Dimensional Structures and Function(part 2)(109页珍藏版)》请在装配图网上搜索。
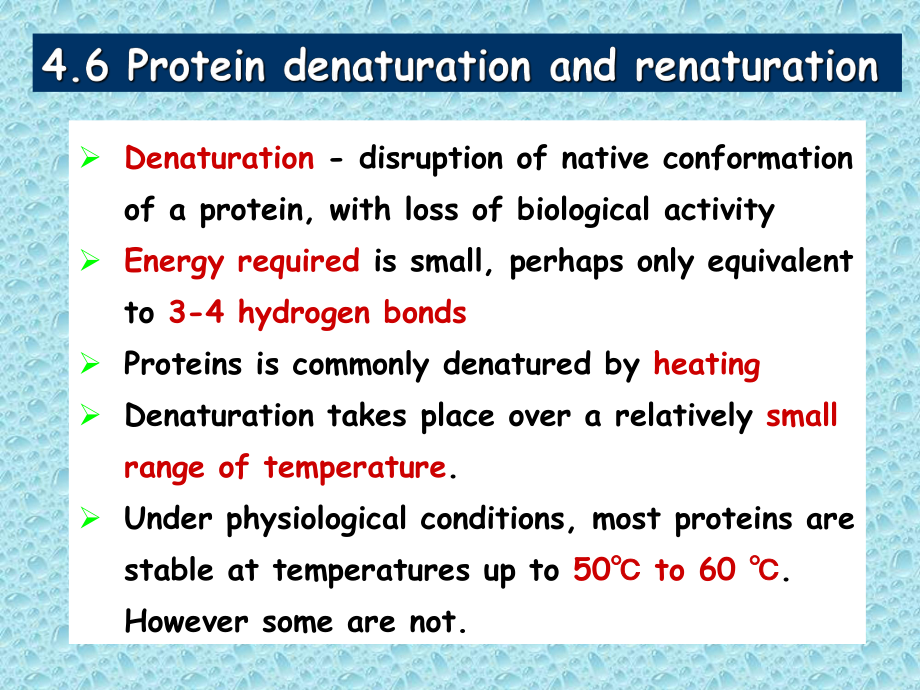
1、 Denaturation - disruption of native conformation of a protein, with loss of biological activity Energy required is small, perhaps only equivalent to 3-4 hydrogen bonds Proteins is commonly denatured by heating Denaturation takes place over a relatively small range of temperature. Under physiologica
2、l conditions, most proteins are stable at temperatures up to 50 to 60 . However some are not. Heat denaturation of ribonuclease A Unfolding monitored by changes in ultraviolet (blue), viscosity (red), optical rotation (green) TmWhat is Tm ? Proteins can also be denatured by two types of chemicals-ch
3、aotropic agents and detergents. For example, urea, guanidinium salts, SDS. Why can the chaotropic agents and detergents result in denaturation of proteins ? The native conformation of some proteins is stabilized by disulfide bonds.Disulfide bridges in bovine ribonuclease A(a) Location of disulfide b
4、ridges(b) Stereo view of Cys-26 and Cys-84a) There are four disulfide bridges in bovine ribonuclease A.(b) The bridge between Cys-26 and Cys 84 is shown in stereo. Can proteins be renatured ? Anfinsens experiments (next page). Conclusion: The conformation of proteins are determined by their primary
5、structure. Protein disulfide isomerase (PDI) and E.coli disulfide isomerase (DsbA) can help misfolded proteins to form correct disulfide bonds.Anfinsens experiments-Denaturation and renaturation of ribonuclease ATreatement of native ribonuclease A (top) with urea in the presence of 2-mercaptoethanol
6、 unfolds the protein and disrupts disulfide bonds to produce reduced, reversibly denatured ribonuclease A (bottom). When the denatured protein is returned to physiological conditions in the absence of 2-mercaptoethanol, it refolds into its native conformation and the correct disulfide bonds form.Pro
7、tein folding and stability Folded proteins occupy a low-energy well that makes the native structure much more stable than alternative conformations. Many proteins can fold spontaneously to this low-energy conformation Folding of proteins is cooperative. Folding of proteins is extremely rapid-in most
8、 cases the native conformation is reached in less than a second.Energy well of protein folding Funnels represent the free energy potential of folding proteinsThe funnels represent the free-energy poteintial of folding proteins. Rather than following a series of folding steps, folding to the final st
9、ructure is illustrated as a parallel process with many possible routes to the lowest energy structure.(a) In this simplified funnel showing two possible pathways to the lowest energy native structure, path A reaches the lowest energy structure directly. In path B the polypeptide enters a local low-e
10、nergy minimum in the process of folding.(b) A more realistic view of the possible free-energy forms of a folding protein includes many local peaks and dips. Proteins folding and stabilization depend on several noncovalent forces and disulfide bond. Hydrophobic effect is the most important for stabil
11、ity of tertiary and secondary structure. Nonpolar side chains associate with each other causing a polypeptide chain to collapse to a molten globuleForces That Stabilize Protein StructureHydrogen bond is very important for stability of secondary structure. They are more stable in hydrophobic envireme
12、nt than in hydrophilic envirement .Contributes to cooperativity of folding Helps stabilize secondary structures and native conformationExamples of hydrogen bondsThe hydrogen bond donors The hydrogen bond donors and acceptors are shown. and acceptors are shown. The most common donor-The most common d
13、onor-acceptor pair in proteins acceptor pair in proteins is the is the amide-carbonylamide-carbonyl, , which is found between which is found between peptide groups. peptide groups. All bonds are All bonds are approximately approximately 0.3nm 0.3nm in in length, with the shortest length, with the sh
14、ortest distance for the shared distance for the shared bonds betwenbonds betwen the most the most similar bond donor similar bond donor acceptor pair.acceptor pair.Van der Waals and Charge-Charge Interactions VDW contacts occur between nonpolar side chains and contribute to the stability of proteins
15、 Charge-charge interactions between oppositely charged side chains in the interior of a protein also may stabilize protein structure Protein Folding Is Assisted by ChaperonesMolecular chaperones increase rate of correct folding and prevent the formation of incorrectly folded intermediates particular
16、ly in very large proteins, smaller proteins need very little assistance.Chaperones can bind to unassembled protein subunits to prevent incorrect aggregation before they are assembled into a multisubunit proteinMost chaperones are heat shock proteins (synthesized as temperature increases)E. coli chap
17、eroninThe core structure of chaperonin consists of two identical rings composed of seven GroEL subunits. Unfolded proteins bind to the central cavity. Bound ATP molecules can be identified by their red oxygen atoms (spacefill). The quaternary structure is shown from (a) the side, and (b) the top. PD
18、B 1DER(c) During folding, the size of the central cavity of one of the rings increases and the end is capped by a protein containing seven GroES subunits. PDB 1AON.(a) (b) Core consists of 2 identical rings (7 GroE subunits in each ring) (c) Protein folding takes place inside the central cavity Chap
19、eronin-assisted protein folding The unfolded polypeptide enters the central cavity of chaperonin, where it folds. The hydrolysis of several ATP molecules is required for chaperonin function.The three dimensional proteins structure is shown in Figure. The lines on the chaperonin cylinder are to repre
20、sent the 7 identical GroEL subunits that make up each ring. Not shown is the end cap composed of GroES subunits. Hypothetical folding pathways are:1. The polypeptide collapses upon itself due to the hydrophobic effect, and elements of secondary structure begin to form;2. Subsequent steps involve rea
21、rrangement of the backbone chain to form characteristic motifs;3. The stable native conformation. Each domain in a multidomain protein folds independently.Hypothetical protein-folding pathways During folding the polypeptide collapses in upon itself due to the hydrophobic effect An intermediate “molt
22、en globule” forms with elements of secondary structure The backbone is rearranged to achieve a stable native conformation1. -Keratin2. -Keratin3. Collagen1. -Keratin:右手右手- 螺旋螺旋 原纤维(左旋的三原纤维(左旋的三股股- 螺旋,直径螺旋,直径2nm)微原纤维(直径微原纤维(直径8nm) 大原纤维(直径大原纤维(直径200nm) 硬角蛋白硬角蛋白含硫量高(二硫键多),如:蹄、爪、角、甲含硫量高(二硫键多),如:蹄、爪、角、甲软
23、角蛋白软角蛋白含硫量低(二硫键少),如:皮肤含硫量低(二硫键少),如:皮肤2. -Keratinsilk fibroin3. Collagen1Collagen is a major protein in connective tissue of vertebrates (25-35% of total protein in mammals)2. Distribution and types: type Itype XII,3Amino acid composition: Gly, Pro, 4-OH-Pro, 3-OH-Pro, 5-OH-Lys (glycoprotein). 4-Hydr
24、oxyproline and 5-hydroxylysine are Formed by enzyme hydroxylation reactions (require vitamin C) after incorporation into collagen Vitamin C deficiency (scurvy) leads to lack of proper hydroxylation and defective triple helix (skin lesions, fragile blood vessels, bleeding gums Unlike most mammals, hu
25、mans cannot synthesize vitamin C 肽链肽链( (helical helical chainschains) ) (非(非螺螺旋,更伸展,左手)旋,更伸展,左手) 三股螺旋三股螺旋(triple (triple helices, helices, procollagenprocollagen) ) three three left-handed helical left-handed helical chains coiled chains coiled around each other around each other in a right-handed i
26、n a right-handed supercoil supercoil (右手超(右手超螺旋缆)螺旋缆) 原胶原分子原胶原分子(Tropocollagen) (Tropocollagen) 胶原(原)纤维胶原(原)纤维 (collagen fibers)(collagen fibers)Stereo view of human Type III collagen triple helix The extended view of the human collagen type III triple helix contains three identical subunits (purple
27、, light blue and green). Three left-handed collagen helices are coiled around one another to form a right-handed supercoil. PDB 1BKVInterchain hydrogen bonding holds the three collagen strands together. The amide hydrogen of a glycine residue in one chain is hydrogen bonded to the caronyl oxygen of
28、a residue, often proline, in an adjacent strand.Multiple repeats of -Gly-X-Y- where X is often proline and Y is often 4-hydroxyprolineGlycine residues are located along central axis of a triple helix (other residues cannot fit)For each -Gly-X-Y- triplet, one interchain H bond forms between amide H o
29、f Gly in one chain and -C=O of residue X in an adjacent chainNo intrachain H bonds exist in the collagen helix Gly , because its small size, is required at the tight junction where the three chains are in contact (motif G-X-X).Interchain H bonding in collagen Amide H of Gly in one chain is H-bonded
30、to C=O in another chainCovalent cross-links in collagenCollagen triple helices aggregate in a staggered fashion to form strong, insoluble fibers. The strength of collage fibrils result from covalent cross-links between collagen molecules. Two allysine residues condense to form an intramolecular cros
31、s-link Glycosylation Tropocollagen assemble into collagen fibers. Crosslinks are formed between lysines through aldol condensation and dehydration. The newly translated collagen is hydroxylated and glycosylated and triple helices are formed. The procollagen triplexes are exported and the globular do
32、mains are cut off to form the tropocollagen The newly translated collagen is hydroxylated and glycosylated and triple helices are formed. The procollagen triplexes are exported and the globular domains are cut off to form the tropocollagen Tropocollagen assemble into collagen fibers. The newly trans
33、lated collagen is hydroxylated and glycosylated and triple helices are formed. The procollagen triplexes are exported and the globular domains are cut off to form the tropocollagen Tropocollagen assemble into collagen fibers. The newly translated collagen is hydroxylated and glycosylated and triple
34、helices are formed. The procollagen triplexes are exported and the globular domains are cut off to form the tropocollagen Crosslinks are formed between lysines through aldol condensation and dehydration. Tropocollagen assemble into collagen fibers. The newly translated collagen is hydroxylated and g
35、lycosylated and triple helices are formed. The procollagen triplexes are exported and the globular domains are cut off to form the tropocollagen Myoglobin is composed of 8 helices Heme prosthetic group binds oxygen His-93 (proximal histidine) is complexed to the iron atom, and His-64 (distal histidi
36、ne) forms a hydrogen bond with oxygen Interior of Mb almost all hydrophobic amino acids Heme occupies a hydrophobic cleft formed by 3 helices and 2 loops Sperm whale oxymyoglobin Oxygen (red) His-93 and His-64 (green)Sperm whale (Physeter catadon) oxymyoglobin consists of eight alpha helices. The he
37、me prosthetic group binds oxygen (red spacefill to right of heme). His-64 (green, right) forms a hydrogen bond with oxygen, and His-93 (green, left) is complexed to the iron atom of the heme. PDB 1A6MThe heme is shown in spacefill in gray and red. Unlike hemoglobin, the structure of myoglobin does n
38、ot change significantly upon binding or release of oxygen.The structure of myoglobinThe structure of heme group Heme (prosthetic group) consists of a tetrapyrrole ring system called protoporphyrin IX complexed with iron.Porphyrin ring provides four of the six ligands surrounding iron atom Oxygen Bin
39、ds Reversibly to Heme Oxymyoglobin - oxygen bearing myoglobin Deoxymyoglobin - oxygen-free myoglobin In oxymyoglobin, six ligands are coordinated to the ferrous ion in octahedral symmetry Oxygen is coordinated between the iron and the imidazole sidechain of His-64 Binding of O2 and myoglobinOxygen-b
40、inding site of whale oxymyoglobinThe heme prosthetic group is The heme prosthetic group is represented by a parallelogram represented by a parallelogram with a nigrogenwith a nigrogen atom at each atom at each corner. The blue dashed lines corner. The blue dashed lines illustrate the octahedral illu
41、strate the octahedral geometry of the coordination geometry of the coordination plex.The hydrogen bond between The hydrogen bond between His-64His-64 and the oxygen molecule and the oxygen molecule is shown in yellow. The is shown in yellow. The presence of His-64 prevents presence of His-64 prevent
42、s oxygen and other diatomic oxygen and other diatomic molecules from binding molecules from binding perpendicularly to the hemeperpendicularly to the heme plane.plane.(distal histidine)(proximal histidine)Proximal histidine residueOxygen-binding in myoglobinFe(II) (orange) lies in the plane of the h
43、eme group. Oxygen (green) is bound to the iron atom and the amino acid side chain of His-64. Val-68 and Phe-43 contirbute to the hydrophobic environment of the oxygen binding site. PDB 1AGMHis-93 complexes on the distal side of the heme from oxygen, making the 5th coordinating nitrogen around the Fe
44、(II). The binding of oxygen in subunits of hemoglobin is almost identical.Conformation change of myoglobin to bind O2Oxygen-binding curve of myoglobinYO2 fractional saturation (Y) is plotted versus the partial pressure of oxygen, pO2 (oxygen concentration)Mb-O2 binding curve is hyperbolic, indicatin
45、g a single equilibrium constant for binding O2蛋白质的结构四级结构The structure of hemoglobinThe quaternary of hemoglobin Conformation change of hemoglobin to bind O2Some salt bridges in hemoglobinConformational changes in a hemoglobin chain induced by oxygenation Oxygen binding to Fe pulls the His toward rin
46、g plane Helix with His shifts position, disrupting some ion pairs between subunits (blue to red position) When the heme iron of a hemoglobin subunit is oxygenated (red), the proximal histidine residue is pulled toward the porphoryn ring. The helix containing the histidine also shifts position, disru
47、pting ion pairs that cross-link the subunits of deoxyhemoglobin.The proximal histidine is His-93 that is complexed to the heme iron. His-64 is sometimes referred to as the distal histidine. The movement of the Fe is only about 0.34nm but leads to a change in oxidation state (as observed in the color
48、 change) and a change in the overall structure of hemoglobin through this initial movement of the proximal histidine.0 20 40 60 80 100 120100806040200Percent O2saturationPartial pressure of oxygen (pO2, mmHg)Muscle inexercisingRelaxingmuscleMyoglobinHemoglobinArteryO2VeinO2Environmental Oxygen Effec
49、ts Binding AffinityAdapted from Garrett & Grisham (1999) Biochemistry (2e) p.480Oxygen-binding curve of hemoglobinComparison of O2-binding to Mb and HbIn this comparison of the fractional saturation (Y) of myoglobin vs. hemoglobin, the saturation (y-axis) is plotted against the pressure of oxygen (p
50、O2) on the x-axis. The oxygen binding curve of myoglobin is hyperbolic with half-saturation (Y=0.5) at an oxygen pressure of 2.8 torr. The oxygen binding cuve of hemoglobin in whole blood is sigmoidal, with a half-saturation pressure of 26 torr. Myoglobin has a greater affinity than hemoglobin for o
51、xygen at all oxygen pressures. In the lungs, where the partial pressure of oxygen is high, hemoglobin is nearly saturated with oxygen. In tissues, where the partial pressure of oxygen is low, oxygen is released from the oxygeneated hemoglobin and transfrerred to myoglobin.Log(Y/1-Y) = n logP(O2) - l
52、og KdYThe effect of H+、CO2 and BPG on hemoglobin binding O21. Bohrs effect2. Carbamate adduct Carbon dioxide is transported from the tissues to the lungs in two ways:(1) Dissolved bicarbonate ions(2) Carbamate adducts of hemoglobin (N-terminal globin residues react with CO2 to form carbamates)3. BPG
53、 can decreate binding ability of Hb to O2Binding of 2,3-BPG to deoxyhemoglobin (-) Charges on 2,3-BPG pair with (+) charges lining the central cavity, stabilizing the DeoxyHb form a-Subunits pink, b-subunits blue, heme groups red The central cavity of deoxyhemoglobin is lined with positively charged
54、 groups that are complementary to the carboxylate and phosphate groups of 2,3-BPG. Both 2,3-BPG and the ion pairs shown help stabilize the deoxy conformation. The alpha subunits are shown in pink, the beta subunits and blue and the heme prosthetic groups in red.The wireframe models of 2,3-BPG and th
55、e charged sidechains illustrate the nature of the interaction of deoxyhemoglobin with 2,3-BPG. In oxyhemoglobin, the center channel is smaller and the charged sidechains are not positioned in such a way as to interact with 2,3-BPG. Therefore, the presence of 2,3-BPG tends to favor the deoxy (T) form
56、.YHbSMolecular diseasessickle cell anemiaPartial primary structure of hemoglobin Partial primary structure of hemoglobin b b chain chainHb-AHb-A: Val-His-Leu-Thr-Pro-Glu-Glu-LysVal-His-Leu-Thr-Pro-Glu-Glu-LysHb-SHb-S: Val-His-Val-His-LeuLeu- -ThrThr-Pro-Val-Pro-Val-GluGlu- -LysLysb b-chain: 1 2 3 4
57、5 6 7 8-chain: 1 2 3 4 5 6 7 8 Humoral immunity Cellular immunity memory B cell effector B cell differentiation memory T cell effector T cell 1. Classification 1. Classification :IgGIgG、IgAIgA、IgMIgM、IgD and IgD and IgEIgEMrMr:150150950 KDa950 KDaC CL Lone of and one of and C CH Hone of one of (IgGI
58、gG)、(IgAIgA)、)、(IgMIgM)、)、(IgDIgD)、)、(IgEIgE)、andand 330 AA330 AA and and 440 AA440 AA and and 210 AA210 AAIgMIgMCDR1, CDR2, CDR3, CDRCDR1, CDR2, CDR3, CDRcomplementaritycomplementarity determining region determining regionFab (fragment of antigen- binding)Fc (fragment crystalizable) 4Biochemical me
59、thods of which base on antigen-antibody interactionBinding of three different antibodies to an antigen(1) Immunodiffusion (2) Immune electrophoresis: (3) Enzyme-linked immunosorbent assay (ELISA)Enzyme Horseradish peroxidase, HRP (辣根过氧化辣根过氧化物酶物酶) Alkaline phosohatase, AP (碱性磷酸酶碱性磷酸酶). HRP的底物的底物 HRPH
60、RP催化过氧化物的氧化反应,最具代表性的过氧化物为催化过氧化物的氧化反应,最具代表性的过氧化物为H H2 2O O2 2,其反应式如下:,其反应式如下:DHDH2 2+ H+ H2 2O O2 2 D+ H D+ H2 2O O上式中,上式中,DHDH2 2为供氢体,为供氢体,H H2 2O O2 2为受氢体。在为受氢体。在ELISAELISA中,中,DHDH2 2一般为无色化一般为无色化合物,经酶作用后成为有色的产物,以便作比色测定。合物,经酶作用后成为有色的产物,以便作比色测定。 常用的供氢体有常用的供氢体有邻苯二胺邻苯二胺(O-phenylenediamine,OPD(O-phenyle
61、nediamine,OPD) )四甲基联苯胺四甲基联苯胺(3,3,5,5-(3,3,5,5-tetramethylbenzidine,TMD)tetramethylbenzidine,TMD)ABTS2,2-azino-di-(3-ethylbenziazobine ABTS2,2-azino-di-(3-ethylbenziazobine s sLfonate-6)Lfonate-6)(4) Western BlotEach hybridoma line can produce pure single antibody, called monoclonal antibody.1234mmmm
62、12341234MonoclonalantibodiesCell fusionSpleen cellsMyelomaxAntiseumAntigenImmunizationA mixture of all Ab1 234BALB/ c12341 234B cell5. Preparation of monoclonal antibody+4.11 Measurement of protein1.1.凯氏定氮法(凯氏定氮法(KjeldahlKjeldahl) 2.2.双缩脲法双缩脲法(biuretbiuret) 3.3.FollinFollin酚法(酚法(LowryLowry) 4.4.染料结合
63、法(染料结合法(BradfordBradford) 5.5.BCABCA法法6.6.紫外吸收法紫外吸收法 1.1. 凯氏定氮法(凯氏定氮法(KjeldahlKjeldahl) 蛋白质含量蛋白质含量6.256.25蛋白蛋白N N含量含量消化:蛋白质消化:蛋白质 + H+ H2 2SOSO4 4 强热和CuSO4 (NHNH4 4)2 2SOSO4 4 + SO + SO2 2+ + CO CO2 2 + H + H2 2O O 蒸馏:(蒸馏:(NHNH4 4)2 2SOSO4 4 + 2NaOH Na + 2NaOH Na2 2SOSO4 4 + 2 H + 2 H2 2O O + 2NH +
64、2NH3 3 2NH 2NH3 3 + 4H + 4H3 3BOBO3 3(NHNH4 4)2B2B4 4O O7 7 + 5H + 5H2 2O O滴定:(滴定:(NHNH4 4)2B2B4 4O O7 7 + 2HCl + 5H + 2HCl + 5H2 2O2NHO2NH4 4Cl Cl + 4 H + 4 H3 3BOBO3 3 2.2.双缩脲法双缩脲法(biuretbiuret) 540nm3. Follin3. Follin酚法(酚法(LowryLowry) FolinFolin- -酚试剂由酚试剂由试剂试剂A A和和试剂试剂B B两部分组成。蛋白质中的肽键首两部分组成。蛋白质中的
65、肽键首先在碱性条件下与酒石酸钾钠先在碱性条件下与酒石酸钾钠- -铜盐溶液(试剂铜盐溶液(试剂A A)起作用生成)起作用生成紫色络合物紫色络合物(类似双缩脲反应)。(类似双缩脲反应)。由于蛋白质中由于蛋白质中酪氨酸、色氨酸酪氨酸、色氨酸的存在,该络合物在碱性条件下的存在,该络合物在碱性条件下进而与试剂进而与试剂B B(磷钼酸和磷钨酸、硫酸、溴等组成)形成(磷钼酸和磷钨酸、硫酸、溴等组成)形成蓝色复蓝色复合物合物,其呈色反应颜色深浅与蛋白质含量成正比(,其呈色反应颜色深浅与蛋白质含量成正比(660nm660nm)。)。可测定蛋白质含量的范围:可测定蛋白质含量的范围:2525250g/mL250g/
66、mL。干扰因素干扰因素:溶液或样品中含有带:溶液或样品中含有带“-CO-NH-CO-NH2 2”、“-CH-CH2 2-NH-NH2 2” ” 、“-CS-NH-CS-NH2 2”基团的化合物,基团的化合物,TrisTris、核酸、蔗糖、硫酸铵、巯基、核酸、蔗糖、硫酸铵、巯基及酚类等化合物时。及酚类等化合物时。Coomassie Brilliant Blue G-250加入蛋白加入蛋白质质干扰因素干扰因素:去污剂,:去污剂,SDS,Triton-100 CBG 是一种指示剂470 nm595 nm 4. Bradford Method:5. BCA5. BCA法法(bicinchonininc(bicinchonininc acid) acid)562nm562nm特点:特点:范围广:范围广:20202000g/ml, 2000g/ml, MicroBCAMicroBCA试剂测定范围是试剂测定范围是0.5-0.5-20g/ml20g/ml。快速:快速:4545分钟内完成测定。分钟内完成测定。 抗干扰:不受离子型和非离子抗干扰:不受离子型和非离子型去污剂影响。型去污剂影响。6. 6. 紫
- 温馨提示:
1: 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
2: 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
3.本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
5. 装配图网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
