 FDA批准的精准医疗诊断体外器械一览表List of Cleared or Approved Companion Diagnostic Devices
FDA批准的精准医疗诊断体外器械一览表List of Cleared or Approved Companion Diagnostic Devices


《FDA批准的精准医疗诊断体外器械一览表List of Cleared or Approved Companion Diagnostic Devices》由会员分享,可在线阅读,更多相关《FDA批准的精准医疗诊断体外器械一览表List of Cleared or Approved Companion Diagnostic Devices(26页珍藏版)》请在装配图网上搜索。
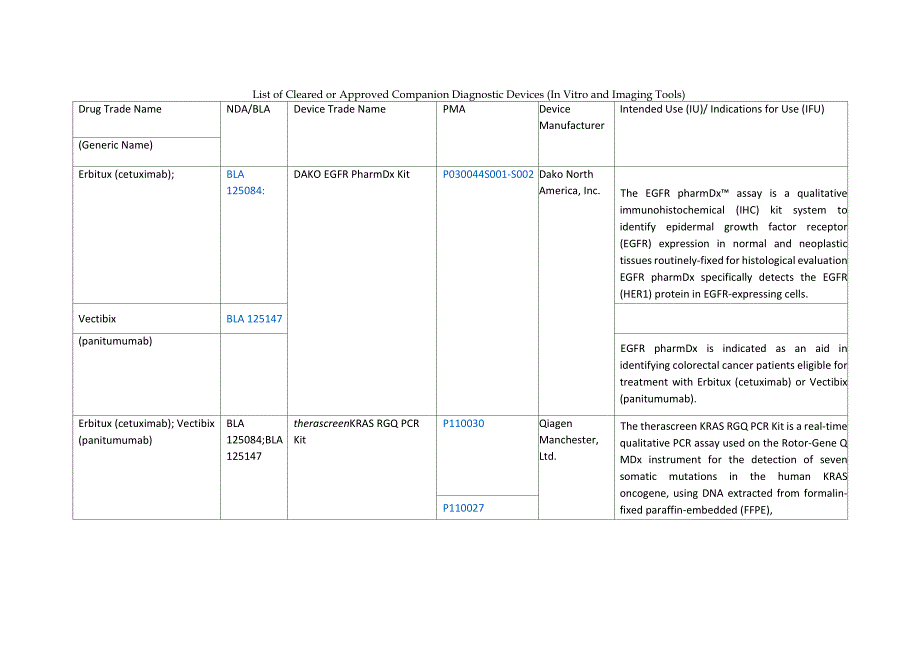
1、List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools)Drug Trade NameNDA/BLADevice Trade NamePMADeviceManufacturerIntended Use (IU)/ Indications for Use (IFU)(Generic Name)Erbitux (cetuximab);BLA125084:DAKO EGFR PharmDx KitP030044S001-S002Dako NorthAmerica, Inc.The EGF
2、R pharmDx assay is a qualitative immunohistochemical (IHC) kit system to identify epidermal growth factor receptor (EGFR) expression in normal and neoplastic tissues routinely-fixed for histological evaluation EGFR pharmDx specifically detects the EGFR (HER1) protein in EGFR-expressing cells.Vectibi
3、xBLA 125147(panitumumab)EGFR pharmDx is indicated as an aid in identifying colorectal cancer patients eligible for treatment with Erbitux (cetuximab) or Vectibix (panitumumab).Erbitux (cetuximab); Vectibix (panitumumab)BLA125084;BLA125147therascreenKRAS RGQ PCRKitP110030QiagenManchester,Ltd.The ther
4、ascreen KRAS RGQ PCR Kit is a real-time qualitative PCR assay used on the Rotor-Gene Q MDx instrument for the detection of seven somatic mutations in the human KRAS oncogene, using DNA extracted from formalin-fixed paraffin-embedded (FFPE),P110027colorectal cancer (CRC) tissue.Thetherascreen KRAS RG
5、Q PCR Kit is intended to aid in the identification of CRC patients for treatment with Erbitux (cetuximab) and Vectibix (panitumumab) based on a KRAS no mutation detected test result.Erbitux (cetuximab); Vectibix (panitumumab)BLA125084;BLA125147The cobas KRAS MutationTestP140023RocheMolecularSystems,
6、 Inc.The cobas KRAS Mutation Test, for use with the cobas 4800 System, is a real-time PCR test for the detection of seven somatic mutations in codons 12 and 13 of the KRAS gene in DNA derivedfromformalin-fixedparaffin-embedded human colorectal cancer (CRC) tumor tissue. The test is intended to be us
7、ed as an aid in the identification of CRC patients for whom treatment with Erbitux (cetuximab) or with Vectibix (panitumumab) may be indicated based on a no mutation detected result. Specimens are processed using the cobas DNA Sample Preparation Kit for manual sample preparation and the cobas z 480
8、analyzer for automated amplification and detection.ExjadeNDA021882FerriscanK124065Resonance Health Analysis Services PtyThe FerriScan R2-MRI Analysis System is intended to measure liver iron concentration to aid in the identification and monitoring of non-transfusiondependent thalassemia(deferasirox
9、)Ltdpatients receiving therapy with deferasirox.GilotrifNDA201292therascreen EGFR RGQ PCRKitP120022Qiagen Manchester, Ltd.The therascreen EGFR RGQ PCR Kit is a real-time PCR test for the qualitative detection of exon 19 deletions and exon 21 (L858R) substitution mutations of the epidermal growth fac
10、tor receptor (EGFR) gene in DNA derivedfromformalin-fixedparaffin-embedded (FFPE) non-small cell lung cancer (NSCLC) tumor tissue. The test is intended to be used to select patients with NSCLC for whom GILOTRIF (afatinib), an EGFR tyrosine kinase inhibitor (TKI), is indicated. Safety and efficacy of
11、 GILOTRIF (afatinib) have not been established in patients whose tumors have L861Q, G719X, S768I, exon 20 insertions, and T790M mutations, which are also detected by the therascreen EGFR RGQ PCR Kit.(afatinib)Specimens are processed using the QIAamp DSP DNA FFPE Tissue Kit for manual sample preparat
12、ion and the Rotor-Gene Q MDx instrument for automated amplification and detection.Gleevec/GlivecNDA021335;DAKO C-KIT PharmDxP040011S001-S002Dako NorthAmerica, Inc.The c-Kit pharmDX assay is a qualitative immunohistochemical (IHC) kit system used on the DakoAutostainer, for the identification of c-ki
13、t protein/CD 117 antigen (c-kit protein) expression in normal and neoplastic formalin-fixed paraffin-embedded tissues for histological evaluation. The c-Kit pharmDX rabbit polyclonal antibodies specifically detect the c-kit protein in CD 117 antigen-expressing cells.(imatinibmesylate)NDA021588The c-
14、Kit pharmDx is indicated as an aid in the differential diagnosis of gastrointestinal stromal tumors (GIST). After diagnosis of GIST, results from c-Kit pharmDx may be used as an aid in identifying those patients eligible for treatmentwithGleevec/Glivec(imatinibmesylate).Results from hematoxylin and
15、eosin (H&E) stains and a panel of antibodies can aid in the differential diagnosis of GIST. Interpretation must be made by a qualified pathologist, within the context of a patients clinical history, proper controls, and other diagnostic tests.HerceptinBLA 103792INFORM HER-2/NEUP940004S001VentanaMedi
16、calSystems, Inc.The Inform Her-2/Neu gene detection system is a fluorescence in situ hybridization (FISH) DNA probe assay that determines the qualitative presence of Her-2/Neu gene amplification on formalin-fixed, paraffin embedded human breast tissue as an aid to stratify breast cancer patients acc
17、ording to risk for recurrence or disease-related death. It is indicated for use as an adjunct to existing clinical and pathologic information currently used as prognostic indicators in the risk stratification of breast cancer in patients who have had a priori invasive, localized breast carcinoma and
18、 who are lymph node-negative.(trastuzumab)HerceptinBLA 103792HER2 CISH PharmDx KitP100024S001-S005DakoDenmark A/SHER2 CISH PharmDx kit is intended for dual-color chromogenic visualization of signals achieved with directly labeled in situ hybridization probes targeting the HER2 gene and centromeric r
19、egion of chromosome 17. The kit is designed to quantitatively determine HER2 gene status in formalin-fixed, paraffin-embedded breast cancer tissue specimens. Red and blue chromogenic signals are generated on the same tissue section for evaluation under bright field microscopy. The CISH procedure is
20、automated usingDakoAutostainer instruments.(trastuzumab)HER2 CISH pharmDx Kit is indicated as an aid in the assessment of patients for whom Herceptin (trastuzumab) treatment is being considered. Results from the HER2 CISH pharmDx Kit are intended for use as an adjunct to the clinicopathologic inform
21、ation currently used for estimating prognosis in stage II, node-positive breast cancer patients.This kit is for in vitro diagnostic (IVD) use only.HerceptinBLA 103792PATHWAY ANTI-HER-2/NEU (4B5) Rabbit Monoclonal Primary AntibodyP990081S001-S028VentanaMedicalSystems, Inc.Ventana Medical Systems PATH
22、WAY Her2 (clone CB11) is a mouse monoclonal antibody intended for laboratory use for the semi-quantitative detection of c-erbB-2 antigen(trastuzumab)insectionsofformalin-fixed,paraffin-embedded normal and neoplastic tissueonaVentanaautomatedimmunohistochemistry slide staining device. It is indicated
23、 as an aid in the assessment of breast cancer patients for whom Herceptin treatment is being considered.HerceptinBLA 103792Bond Oracle Her2 IHC SystemP090015S001LeicaBiosystemsThe Bond Oracle Her2 IHC system is a semi-quantitative immunohistochemical (IHC) assay to determine Her2 (human epidermal gr
24、owth factor receptor 2) oncoprotein status in formalin-fixed, paraffin-embedded breast cancer tissue processed for histological evaluation following automated staining on the bond-max slide staining instrument. The Bond Oracle Her2 IHC system is indicated as an aid in the assessment of patients for
25、whom herceptin (trastuzumab) treatment is being considered.(trastuzumab)HerceptinBLA 103792SPOT-LIGHT HER2 CISH KitP050040S001-S003Life Technologies, Inc.For In Vitro Diagnostic Use.(trastuzumab)The SPOT-Light HER2 CISH Kit is intended to quantitatively determine HER2 gene amplificationinformalin-fi
26、xed,paraffin-embedded (FFPE) breast carcinoma tissue sections using Chromogenic In Situ Hybridization(CISH)andbrightfieldmicroscopy.This test should be performed in a histopathology laboratory.The SPOT-Light HER2 CISH Kit is indicated as an aid in the assessment of patients for whom Herceptin (trast
27、uzumab) treatment is being considered. The assay results are intended for use as an adjunct to the clinicopathological information currently being used as part of the management of breast cancer patients. Interpretation of test results must be made within the context of the patients clinical history
28、 by a qualified pathologist.HerceptinBLA 103792INSITE HER-2/NEU KITP040030BiogenexLaboratories,Inc.InSite Her-2/neu Mouse Monoclonal Antibody (Clone C1B11) kit is intended for In Vitro Diagnostic use in Immunohistochemistry (IHC) assays to semi-quantitatively localize by light(trastuzumab)microscopy
29、 the over-expression of Her-2/neu (i.e.,c-erbB-2)informalin-fixed,paraffin-embedded normal and neoplastic tissue sections. InSite Her-2/neu is indicated as an aid in the assessment of breast cancer patients for whom Herceptin (Trastuzumab) therapy is being considered. Clinical interpretationofInSite
30、Her-2/neuimmunostaining results (absence or presence; semi-quantitative intensity score) should be complemented by appropriate controls and morphological tissue analysis and be evaluated by a qualified pathologist within the context of patient clinical history and other diagnostic results.HerceptinB
31、LA 103792INFORM HER2 DUAL ISH DNAProbe CocktailP100027S001-S017VentanaMedicalSystems, Inc.The INFORM HER2 Dual ISH DNA Probe Cocktail is intended for use in determining HER2 gene status by enumeration of the ratio of the HER2 gene to Chromosome 17. The HER2 and Chromosome 17 probes are detected usin
32、g two color chromogenic in situ hybridization(ISH)informalin-fixed,paraffin-embedded human breast cancer tissue specimensfollowingstainingonVentanaBenchMark XT automatedslidestainers (using NexES software), by lightmicroscopy. The INFORM HER2 Dual ISH DNA Probe Cocktail is indicated as an aid in the
33、 assessment of patients for whom Herceptin (trastuzumab) treatment is being considered.(trastuzumab)This product should be interpreted by a qualified reader in conjunction with histological examination, relevant clinical information, and proper controls.This reagent is intended for in vitro diagnost
34、ic (IVD) use.HerceptinBLA 103792PATHVYSION HER-2 DNAProbe KitP980024S001-S012AbbottMolecularInc.The PathVysion HER-2 DNA Probe Kit (PathVysion Kit) is designed to detect amplification of the HER-2/neu gene via fluorescence in situ hybridization (FISH) in formalin-fixed, paraffin-embedded human breas
35、t cancer tissue specimens. Results from the PathVysion Kit are intended for use as an adjunct to existing clinical and pathologic information currently used as prognostic factors in stage II, node-positive breast cancer patients. The PathVysion Kit is further indicated as an aid to predict disease-f
36、ree and overall survival in patients with stage II, node positive breast cancer treated with adjuvant cyclophosphamide,doxorubicin,and5-fluorouracil (CAF) chemotherapy. The Pathvysion Kit is indicated as an aid in the assessment of patients for whom herceptin (trastuzumab) treatment is being conside
37、red (see herceptin package insert).HerceptinBLA103792;HERCEPTESTP980018S001-S018DakoDenmark A/SFor in vitro diagnostic use.(trastuzumab);BLA 125409Perjeta (pertuzumab);HercepTestisasemi-quantitativeimmunocytochemical assay to determine HER2 protein overexpression in breast cancer tissues routinely p
38、rocessed for histological evaluation and formalin-fixed, paraffin-embedded cancer tissue from patients with metastatic gastric or gastroesophageal junction adenocarcinoma. HercepTest is indicated as an aid in the assessment of breast and gastric cancer patients for whom Herceptin (trastuzumab) treat
39、ment is being considered and for breast cancer patients for whom PERJETA (pertuzumab) treatment orKADCYLA(ado-trastuzumabemtansine) treatment is being considered (see Herceptin, PERJETA and KADCYLA package inserts).Kadcyla(ado-trastuzumabemtansine)NOTE for breas t cancer oAllyof the patients in the
40、Herceptin clinical trials were selected using an investigational immunocytochemical clinical trial assay (CTA). None of the patients in those trials were selected using the HercepTest. The HercepTest was compared to the CTA on an independent set of samples and found to provide acceptably concordant
41、results. The actual correlation of theHercepTest to Herceptin clinical outcome has not been established.NOTE for gastric cancer only: All of the patients in the phase III BO18255 (ToGA) study sponsored by Hoffmann-La Roche were selected using DakoHercepTest (IHC) and Dako HER2 FISH pharmDx Kit (FISH
42、). However, enrollment in the BO18255 study was limited to patients whose tumors were HER2 protein overexpressing (IHC 3+) or gene amplified (FISH+; HER2/CEN-17 ratio 2.0). No patients were enrolled whose tumors were not gene amplified but HER2 protein weakly to strongly overexpressing FISH(-)/IHC 2
43、+, therefore it is unclear if patients whose tumors are not gene amplified but HER2 protein overexpressing i.e., FISH(-), IHC 2+ or 3+ will benefit from Herceptin treatment. The study also demonstrated that gene amplification and protein overexpression (IHC) are not as correlated as with breast canc
44、er, therefore asingle method should not be used to determine HER2 status.HerceptinBLA103792;HER2 FISH PharmDx KitP040005S001-S010DakoDenmark A/SHER2 IQFISH pharmDx is a direct fluorescence in situ hybridization (FISH) assay designed to quantitativelydetermineHER2geneamplificationinformalin-fixed,par
45、affin-embedded (FFPE) breast cancer tissue specimens and FFPE specimens from patients with metastatic gastric or gastroesophageal junction adenocarcinoma.(trastuzumab);BLA 125409Perjeta (pertuzumab);HER2 IQFISH pharmDx is indicated as an aid in the assessment of breast and gastric cancer patients fo
46、r whom Herceptin (trastuzumab) treatment is being considered and for breast cancerpatients for whomPerjeta(pertuzumab)orKadcyla(ado-trastuzumabemtansine) treatment is being considered (see Herceptin, Perjeta and Kadcyla package inserts).Kadcyla(ado-trastuzumabemtansine)For breast cancer patients, re
47、sults from the HER2 IQFISH pharmDx are intended for use as an adjunct to the clinicopathologic information currently used for estimating prognosis in stage II, node-positive breast cancer patients.Iressa (gefitinib)NDA206995therascreen EGFR RGQ PCRKitP120022S001QiagenManchester,Ltd.The therascreen E
48、GFR RGQ PCR Kit is a real-time PCR test for the qualitative detection of exon 19 deletions and exon 21 (L858R) substitution mutations of the epidermal growth factor receptor (EGFR) gene in DNA derivedfromformalin-fixedparaffin-embedded (FFPE) non-small cell lung cancer (NSCLC) tumor tissue. The test
49、 is intended to be used to select patients with NSCLC for whom GILOTRIF (afatinib) or IRESSA (gefitinib), EGFR tyrosine kinase inhibitors (TKIs), is indicated. Safety and efficacy of GILOTRIF (afatinib) and IRESSA (gefitinib) have not been established in the patients whose tumors have L861Q, G719X,
50、S768I, exon 20 insertions, and T790M mutations, which are also detected by thetherascreen EGFR RGQ PCR Kit.Specimens are processed using the QIAamp DSP DNA FFPE Tissue Kit for manual sample preparation and the Rotor-Gene Q MDx instrument for automated amplification and detection.KEYTRUDA(pembrolizum
51、ab)BLA125514/S-5PD-L1 IHC 22C3 pharmDxP150013Dako, NorthAmerica, Inc.PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung canc
52、er (NSCLC) tissue using EnVision FLEX visualization system on Autostainer Link 48. PD-L1 protein expression is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining. The specimen should be consideredPD-L1 positiv
53、e if TPS 50% of the viable tumor cells exhibit membrane staining at any intensity.PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with KEYTRUDA (pembrolizumab).Lynparza (olaparib)NDA206162BRACAnalysisCDxP140020Myriad Genetic Laboratories, Inc.BRACAnalysisCDx
54、 is an in vitro diagnostic device intended for the qualitative detection and classification of variants in the protein coding regions and intron/exon boundaries of the BRCA1 and BRCA2 genes using genomic DNA obtained from whole blood specimens collected in EDTA. Single nucleotide variants and small
55、insertions and deletions (indels) are identified by polymerase chain reaction (PCR) and Sanger sequencing. Large deletions and duplications in BRCA1 and BRCA2 are detected using multiplex PCR. Results of the test are used as an aid in identifying ovarian cancer patients with deleterious or suspected
56、deleterious germline BRCA variants eligible for treatment with Lynparza (olaparib). This assay is for professional use only and is to be performed only at Myriad Genetic Laboratories, a single laboratory site located at 320 Wakara Way, Salt Lake City, UT 84108.Mekinist(tramatenib);Tafinlar (dabrafen
57、ib)NDA204114;THxID BRAF KitP120014bioMerieux Inc.The THxID BRAF kit is an In Vitro Diagnostic device intended for the qualitative detection of the BRAF V600E and V600K mutations in DNA samples extracted from formalin-fixed paraffin-embedded (FFPE) human melanoma tissue. The THxID BRAF kit is a real
58、-time PCR test on the ABI 7500 Fast Dx system and is intended to be used as an aid in selecting melanoma patients whose tumors carry the BRAF V600E mutation for treatment with dabrafenib Tafinlar and as an aid in selecting melanoma patients whose tumors carry the BRAF V600E or V600K mutation for tre
59、atment with trametinib Mekinist.NDA202806Tagrisso (osimertinib)NDA208065cobas EGFR Mutation Test v2P120019S007RocheMolecularSystems, Inc.The cobas EGFR Mutation Test v2 is a real-time PCR test for the qualitative detection of defined mutations of the epidermal growth factor receptor (EGFR) gene in D
60、NA derived from formalin-fixed paraffin-embedded tumor tissue (FFPET) from non-small cell lung cancer(NSCLC) patients. The test is intended to aid in identifying patients with NSCLC whose tumors have defined EGFR mutations and for whom safety and efficacy of a drug have been established as follows:T
61、arceva (erlotinib) - Exon 19 deletions andL858RTagrisso (osimertinib) - T790MDrug safety and efficacy have not been established for the following EGFR mutations also detected by the cobas EGFR Mutation Test v2:Tarceva (erlotinib) -G719X, exon 20insertions, T790M, S768I and L861QTagrisso (osimertinib
62、) - G719X, exon 19 deletions, L858R, exon 20 insertions, S768I, and L861QFor manual sample preparation, FFPET specimens are processed using the cobas DNA Sample Preparation Kit and the cobas z480 analyzer is used for automated amplification and detection.Tarceva (erlotinib)NDA021743cobas EGFR Mutati
63、on TestP120019S001-S004RocheMolecularSystems, Inc.The cobas EGFR Mutation Test is a real-time PCR test for the qualitative detection of exon 19 deletions and exon 21 (L858R) substitution mutations of the epidermal growth factor receptor (EGFR) gene in DNA derived from formalin-fixed paraffin-embedded (FFPET) human non-small cell lung cancer (NSCLC) tumor tissue. The test is intended to be used as an aid in selecting patients with NSCLC for whom Tarceva (erlotinib)
- 温馨提示:
1: 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
2: 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
3.本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
5. 装配图网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
