 化学原理Chemistry课件post7kinetics
化学原理Chemistry课件post7kinetics


《化学原理Chemistry课件post7kinetics》由会员分享,可在线阅读,更多相关《化学原理Chemistry课件post7kinetics(31页珍藏版)》请在装配图网上搜索。
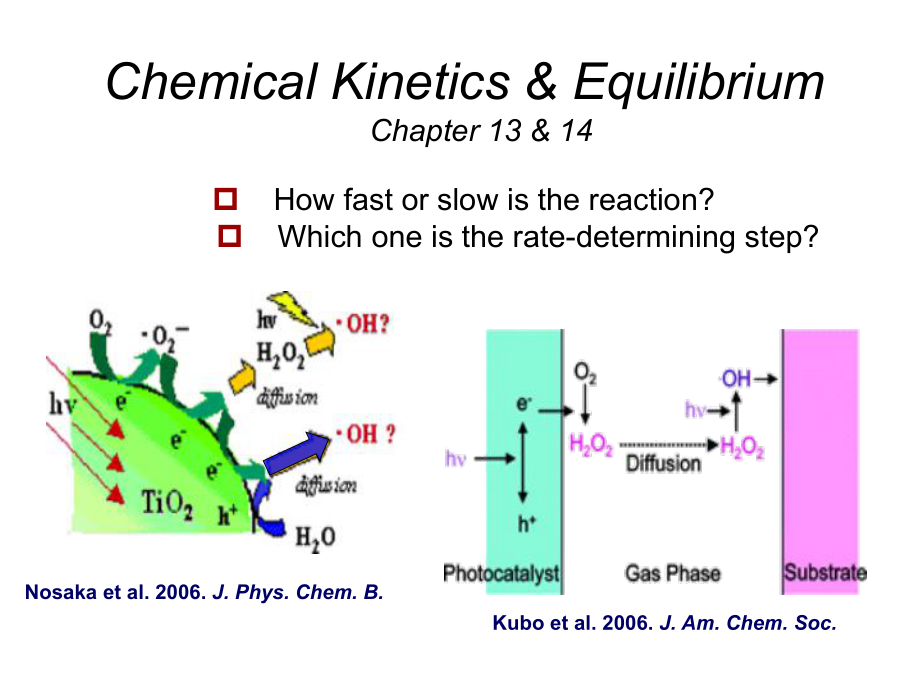
1、Chemical Kinetics&Equilibrium Chapter 13&14p How fast or slow is the reaction?p Which one is the rate-determining step?Nosaka et al.2006.J.Phys.Chem.B.Kubo et al.2006.J.Am.Chem.Soc.1.Reaction rate:rate=-DADtrate=DCDtaA+bB cC+dDrate=-DADt1a=-DBDt1b=DCDt1c=DDDt1dCH4(g)+2O2(g)CO2(g)+2H2O(g)rate=-DCH4Dt
2、=-DO2Dt12=DH2ODt12=DCO2DtBr2(aq)+HCOOH(aq)2Br-(aq)+2H+(aq)+CO2(g)393 nmlightDetector393 nmBr2(aq)I0ItUV-visible absorption spectroscopic spectrumA:absorbance e e :molar coefficient(cm-1.M-1)l l:light path length(cm)C:concentration(M)It=I0 e-e e l ClcIIAte0lnLambert-Beer Lawaverage rate=-DBr2Dt=-Br2f
3、inal Br2initialtfinal-tinitialslope oftangentslope oftangentslope oftangentinstantaneous rate=a rate for specific instance in timeInitial rate is the rate measured in the initial period of time,so as to avoid possible interference from reaction intermediates or products.rate=k Br2k=3.50 x 10-3 s-1 r
4、ate constantSlope=3.50 x 10-3 s-1This reaction is called as first-order to Br2.This is called the Rate law.aA+bB cC+dDRate=k Ax ByIt means that the reaction is the(x+y)th order,the xth in A,and the yth in B.Note that:p The rate laws are always determined by experiment.p X or y can be any value,but i
5、t does not necessarily equal to a or b(x a,y b)In general,F2(g)+2ClO2(g)2FClO2(g)As F2 is constant,quadruples ClO2,the rate is quadrupled.y=1Assume:rate=k F2x ClO2yAs ClO2 is constant,doubles F2,the rate is also doubled.y=1Therefore:rate=k F2x ClO2yDetermine the rate law and calculate the rate const
6、ant for the following reaction from the following data:S2O82-(aq)+3I-(aq)2SO42-(aq)+I3-(aq)ExperimentS2O82-I-Initial Rate(M/s)10.080.0342.2 x 10-420.080.0171.1 x 10-430.160.0172.2 x 10-4Assume:rate=k S2O82-x I-yWe see that x=1;y=1k=rateS2O82-I-=2.2 x 10-4 M/s(0.08 M)(0.034 M)=0.08/Msrate=k S2O82-I-T
7、he reaction is the 2rd order.First-Order ReactionsA productrate=-DADt=k ADADt=k A-A0 and A are the concentration of A at t=0 and t=t,respectively.A=A0exp(-kt)The half-lifelnA0A0/2k=tln2k=lnA=lnA0-ktThe reaction 2A B is first order in A with a rate constant of 2.8 x 10-2 s-1 at 800C.How long will it
8、take for A to decrease from 0.88 M to 0.14 M?lnA=lnA0-ktt=lnA0 lnAk=66 sA0=0.88 MA =0.14 MlnA0Ak=ln0.88 M0.14 M2.8 x 10-2 s-1=What is the half-life of the reactant A in this reaction?tln2k=s 25s 028.0693.01-Second-Order ReactionsA productrate=-DADtDADt=k A2-A0 and A is the concentration of A at time
9、 t=0,and t=t,respectively.1A=1A0+ktt=1kA0IfZero-Order ReactionsA productrate=k A0=kA=A0-ktDADt=k-t=A02kSummary of Common Reaction KineticsOrderRate LawConcentration-Time EquationHalf-Life012rate=krate=k Arate=k A2lnA=lnA0-kt1A=1A0+ktA=A0-kttln2k=t=A02kt=1kA02.Effect of Temperature lnk=-1TB +CThe rat
10、e of most chemical reactions increases as the temperature rises.The experimental data of such effect can be well fitted to the following equation.p The value of Ea varies from reaction to reaction(40 400 kJ/mol).p Larger the value of Ea,stronger the temperature effect on the reaction rate.Ea is the
11、minimum amount of energy required to react.It is now called as activation energy.A is the frequency factor.R is the gas constant(8.314 J/Kmol).T is the absolute temperature(K).22111 2lnaEkTTkRTTIn 1888,Arrhenius proposed an empirical equation to describe the temperature effect.(-)aERTkA eln ln aEkAR
12、THow to explain the concentration and temperature effect?(1)The collision modelThe reacting molecules must collide with one another.The colliding molecule should have a certain minimum energy(Ea).Eap As temperature increases,the fraction of active molecules increases.So the reaction rate increases w
13、ith T.p As concentration or pressure increases,the total number of active molecules increases,and so the reaction rate increases.The colliding molecules must form an activated complex in a preferable direction.In this transition state,the effective energy transfer and chemical bond rearrangement occ
14、ur at one time.(2)Transition state modelCO2(g)+NO(g)CO(g)+NO2(g)OCONOProgress of reactionPotential energy kJEa=134 kJEa=360 kJD DH=-226 kJExothermic ReactionEndothermic ReactionA+B C+D3.Reaction MechanismsAt molecular level,the observed chemical reaction(called also as complicated reaction)is actual
15、ly composed of a series of simple elementary steps or reactions.Elementary step or reaction is the step or reaction that really occurs during the chemical reaction.2NO(g)+O2(g)2NO2(g)N2O2 is detected during the reaction,called as the intermediateElementary step:NO+NO N2O2Elementary step:N2O2+O2 2NO2
16、Overall reaction:2NO+O2 2NO2+Complicated reaction:Based on this information,one proposed the following reaction mechanism:Unimolecular reactionA productsrate=k ABimolecular reactionA+B productsrate=k ABBimolecular reactionA+A productsrate=k A2For elementary steps,the rate raw can directly be written
17、 as.Writing plausible reaction mechanisms:The sum of the elementary steps must give the overall balanced equation for the reaction.The rate-determining step should predict the same rate law that is determined experimentally.The rate-determining step is the slowest step in the sequence of steps leadi
18、ng to product formation.The experimental rate law for the reaction between NO2 and CO to produce NO and CO2 is rate=kNO22.The reaction is believed to occur via two steps:Step 1:NO2+NO2 NO+NO3Step 2:NO3+CO NO2+CO2What is the equation for the overall reaction?NO2+CO NO+CO2What is the intermediate?NO3W
19、hat can you say about the relative rates of steps 1 and 2?rate=kNO22 is the rate law for step 1 so step 1 must be slower than step 24.Chemical equilibriumequilibriumN2O4(g)2NO2(g)Rate(+)Rate(-)At the equilibrium,p Rate(+)=Rate(-)p Both N2O4 and NO2 remain constant.p it is a dynamic equilibriumKc=NO2
20、2N2O4Equilibrium constantKp=NO2P2N2O4PKc KpHere P(pa),Po=101 325 paHere C(mol/L),Co=1 mol/LaA+bB cC+dD cdCDCabABCCKCCIt is quite often written as cdCDPabABPPKPP0000cdCDCabABCCCCKCCCC 0000cdCDPabABPPPPKPPPP If pressure is in a unit of atm,Kp=Kc(RT)DnFor a gas reaction,here Dn=(c+d)(a+b)The equilibriu
21、m concentrations for the reaction between carbon monoxide and molecular chlorine to form COCl2(g)at 740C are CO=0.012 M,Cl2=0.054 M,and COCl2=0.14 M.Calculate the equilibrium constants Kc and Kp.CO(g)+Cl2(g)COCl2(g)Kc=COCl2COCl2=0.140.012 x 0.054=220Kp=Kc(RT)DnDn=1 2=-1R=0.0821T=273+74=347 KKp=220 x
22、(0.0821 x 347)-1=7.714.2The concentration of solids and pure liquids are taken as a unit,and not included in the K expression.14.2CH3COOH(aq)+H2O(l)CH3COO-(aq)+H3O+(aq)Kc=CH3COO-H3O+CH3COOHH2OSince H2O=constantCaCO3(s)CaO(s)+CO2(g)Kc=CaOCO2CaCO3Since CaCO3=constant CaO=constantKc=CO2Kp=PCO2Kc=CH3COO
23、-H3O+CH3COOH=Kc H2OConsider the following equilibrium at 295 K:The partial pressure of each gas is 0.265 atm.Calculate Kp and Kc for the reaction?NH4HS(s)NH3(g)+H2S(g)Kp=PNH3H2SP=0.265 x 0.265=0.0702Kp=Kc(RT)DnKc=Kp(RT)-DnDn=2 0=2T=295 KKc=0.0702 x(0.0821 x 295)-2=1.20 x 10-414.2The factors influenc
24、ing the chemical equilibrium:(1)Increasing A or B will shift the reaction to the right,until a new equilibrium is established to keep K.aA+bB cC+dD(2)Increasing system pressure will shift the reaction to the right if Dn 0,or to the left if Dn 0,or to the left if DH 0.If an external stress is applied
25、 to a system at equilibrium,the system will make adjustment to offset such stress,consequently resulting into a new equilibrium position.Le Chteliers Principleincreasing or decreasing the reaction rate,without loss of catalyst,only due to a change in the reaction pathway.5.catalysisk=A exp(-Ea/RT)Ea
26、k Catalyst does not change the equilibrium constant,and also does not shift the equilibrium.Catalyst lowers both Ea of forward and reverse reactions.Homogeneous catalysisheterogeneous catalysisN2(g)+3H2(g)2NH3(g)Fe/Al2O3/K2OcatalystCO+Unburned Hydrocarbons+O2CO2+H2Ocatalyticconverter2NO+2NO22N2+3O2catalyticconverterExercises:13.3713.4213.7313.114 14.6214.8817.1 kJ2.1 kJ0.4 kJO3+OO3+O+ClD DH=-392 kJProgress of reaction2O22O2+ClO2CFCl3UVUVDepletion of Ozone in the stratosphere Chapter 17
- 温馨提示:
1: 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
2: 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
3.本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
5. 装配图网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
