 ISO14971-2007不安全管理报告模板-中英对照
ISO14971-2007不安全管理报告模板-中英对照


《ISO14971-2007不安全管理报告模板-中英对照》由会员分享,可在线阅读,更多相关《ISO14971-2007不安全管理报告模板-中英对照(16页珍藏版)》请在装配图网上搜索。
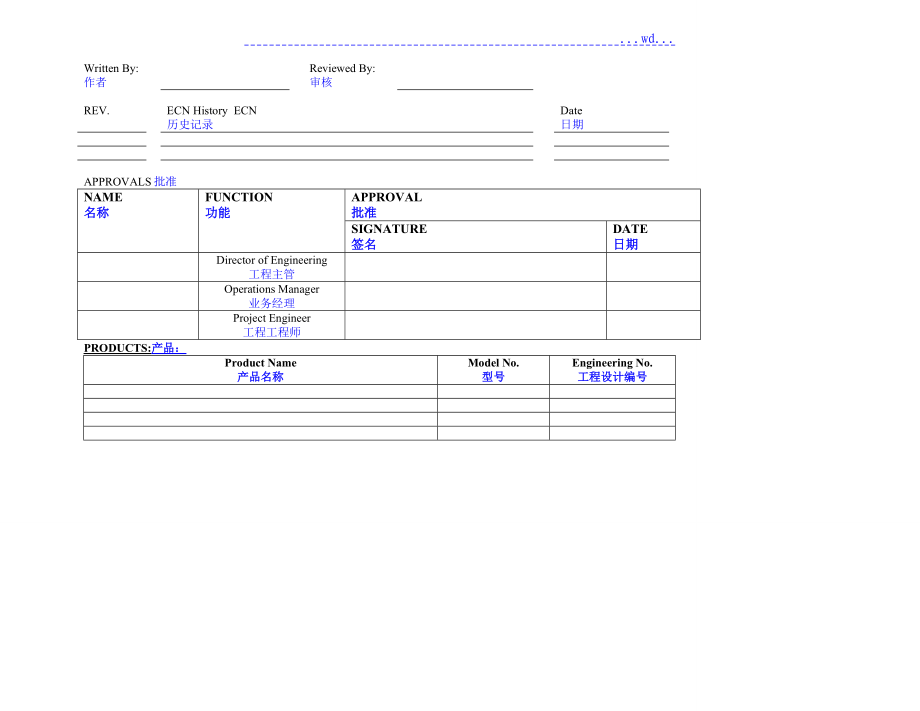
1、 .wd.Written By:作者Reviewed By:审核REV. ECN History ECN历史记录Date日期APPROVALS批准NAME名称FUNCTION功能APPROVAL 批准SIGNATURE 签名DATE日期Director of Engineering工程主管Operations Manager业务经理Project Engineer工程工程师PRODUCTS:产品:Product Name产品名称Model No.型号Engineering No.工程设计编号Table of Contents目录TABLE-E.1 ENERGY HAZARDS表格E-1兼容危害
2、性5ELECTROMAGNETIC ENERGY电磁兼容性5RADIATION ENERGY辐射电磁波5MECHANICAL ENERGY机械兼容性6TABLE-E.1 BIOLOGICAL AND CHEMICAL HAZARDS表格E-1生化危害性7BIOLOGICAL生物学制品7CHEMICAL化学制品7BIOCOMPATABILITY8TABLE-E.1 OPERATIONAL HAZARDS表格E-1操作危害性8FUNCTION功能8USE ERROR使用误差9TABLE-E.1 INFOMATION HAZARDS表格E-19LABELING标号9OPERATATING INSTR
3、UCTIONS操作使用说明10WARNINGS警告11TABLE-E.2 INITIATING EVENTS AND CIRCUMSTANCES表格E-2 启动结果与环境12INCOMPLETE REQUIREMENTS:不完全12MANUFACTURING PROCESSES生产程序12TRANSPORT AND STORAGE运输和储存13ENVIROMANTAL FACTORS环境因素13CLEANING, DISENFECTING, AND STERIALIZATION14DISPOSAL AND SCRAPPING 处理和报废14FORMULATION简要表述15HUMAN FACT
4、ORS人为因素15FAILURE MODES故障模式18Conclusion:结论19To be completed per instructions SOP-XX-XXRisk Management, current revision, in conjunction with standard ISO 14971 and MDD 93/42/EEC对于完成的标准操作程序中的风险管理和近期修正中的每项说明都应与标准的ISO 14971 以及MDD 93/42/EEC保持一致Note: ALL Boxes in matrix must have an entry whether applicab
5、le or not.注:所有的方框, 必须要做登记适用与否(Use N/A for boxes which are not applicable, The ACTION TAKEN Box must be filled out for an explanation “Why it is not Applicable)使用N/A来判定方框哪个是不适用的。采用的方框必须填写明确的解释,“为什么不能用PROBABILITY OF OCCURRENCE发生的 可能性PROBABILITY RANGE概率等级DESCRIPTION描述FREQUENT发生频繁 10-3OCCURRING OFTEN OR
6、 REPEATEDLY经常发生或反复发生PROBABLE很可能的 10-3 and 10-4REASONABLY LIKELY TO OCCUR有时候可能发生OCCASIONAL偶发的 10-4 and 10-5IRREGULAR OCCURRENCE, INFREQUENT不规律发生, 不常见的REMOTE很少发生的 10-5 and 10-6NOT LIKELY TO OCCUR不可能发生IMPROBABLE不可能的 10-6 UNLIKELY TO EVER OCCUR不大可能发生SEVERITY严重程度DESCRIPTION描述CATASTROPHIC致命的RESULTS IN PAT
7、IENT DEATH导致患者死亡CRITICAL不安全的RESULTS IN PERMANENT IMPAIRMENT OR LIFE-THREATENING INJURY导致永久性损伤或危及生命的伤害SERIOUS严重的RESULTS IN INJURY OR IMPAIRMENT REQUIRING PROFESSIONAL MEDICAL INTERVENTION导致伤害或需要医务人员介入MINOR次要的LOW RISK FAILURE NOT EXPECTED TO CONTRIBUTE TO AN INJURY对比低的风险故障对之造成的伤害不可预知NEGLIGIBLE微小的INSIG
8、NIFICANT FAILURE NOT SERIOUS ENOUGH TO CONTRIBUTE TO AN INJURY可以忽略的故障缺乏以促成严重伤害PROBABILITY OFOCCURRENCE发生的可能性SEVERITY CATEGORIES严重程度分类NEGLIGIBLE忽略不计MINOR次要SERIOUS严重CRITICAL不安全临界CATASTROPHIC致命FREQUENT频繁147531PROBABLE可能169752OCCASIONAL偶发1812964REMOTE很少191513118IMPROBABLE不可能2017151310HAZARD RISK INDEX不安
9、全指数ACCEPTANCE CRITERIA承受标准1 TO 5UNACCEPTABLE不能承受6 TO 9UNDESIRABLE. WRITTEN & REVIEWED DECISION TO PROCEED著明不合格或需要进一步审核10 TO 16ACCEPTABLE UPON COMPLETION OF QUALITY 可承受的在质量达标上线ASSURANCE/REGULATORY REVIEW保险但要调制审核17 TO 20ACCEPTABLE WITHOUT REVIEW合格不需要审查Examples of hazards, foreseeable sequences of even
10、ts and hazardous situationsfrom EN ISO 14971:2007 Annex E不安全的例子, 可预测事情进展以及不安全情况TABLE-E.1 ENERGY HAZARDS电磁波危害ELECTROMAGNETIC ENERGY电磁兼容性HAZARD危害因素APPLICABLE适用的POTENTIALFAILURE潜在故障CAUSE OFFAILURE故障原因EFFECTS OF FAILURE故障影响RESPONSE反响RISKINDEX不安全指数ACTION TAKEN采用的措施Line Voltage线路电压Yes NoDesign 设计 Label 标签
11、Enclosure Leakage current附件漏电Yes NoDesign LabelEarth Leakage current接地漏电Yes NoDesign LabelPatient Leakage current耐漏电Yes NoDesign LabelElectric Fields电场Yes NoDesign LabelMagnetic Fields磁场Yes NoDesign LabelRADIATION ENERGY电磁波辐射HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERI
12、SKINDEXACTION TAKENIonizing radiation致电离辐射Yes NoDesign LabelNon-ionizing radiation非电离辐射Yes NoDesign LabelMECHANICAL ENERGYHAZARDAPPLICABLEPOTENTIALFAILURCAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENGravity - Falling地心引力-下落Yes NoDesign LabelGravity suspended masses地心引力被悬浮的质量Yes NoDes
13、ign LabelVibration振动Yes NoDesign LabelStored Energy储存的能量Yes NoDesign LabelMoving Parts移动的部件Yes NoDesign LabelTorsion, shear and tensile forceYes NoDesign LabelMoving and positioning of patient患者移动和转动Yes NoDesign LabelAcoustic energy - ultrasonic energy声能-超声波能量Yes NoDesign LabelAcoustic energy - infr
14、asound energy 超低声波能Yes NoDesign LabelAcoustic energy - sound 声音Yes NoDesign LabelHigh pressure fluid injection高压液体注入Yes NoDesign LabelTABLE-E.1 BIOLOGICAL AND CHEMICAL HAZARDS生物化学危害BIOLOGICAL生物学的HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENBacteria细菌Y
15、es NoDesign LabelViruses病毒Yes NoDesign LabelOther agents (e.g. poisons)其它Yes NoDesign LabelRe- or cross-infection交差感染Yes NoDesign LabelCHEMICAL: Exposure of airway, tissues,environment or property e.g. to foreign materials化学因素:导气管暴露、组织、环境、外来材料HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF
16、 FAILURERESPONSERISKINDEXACTION TAKENAcids or alkalis酸碱Yes NoDesign LabelResidues残留物Yes NoDesign LabelContaminants污染物Yes NoDesign LabelAddictives or processing aids加工助剂Yes NoDesign LabelCleaning, disinfecting or testing agents清洁消毒或检测制剂Yes NoDesign LabelDegradation products降解产物Yes NoDesign LabelMedic
17、al gasses医疗气体Yes NoDesign LabelAnesthetic products麻醉制剂Yes NoDesign LabelBIOCOMPATABILITY, Toxicity of chemical constituents, e.g.化学毒性成分HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENAllergenicity/ irritancy过敏性/刺激Yes NoDesign LabelPyrogenicity (induce fe
18、ver)发热性( 导致发烧)Yes NoDesign LabelTABLE-E.1 OPERATIONAL HAZARDS操作危害FUNCTION功能HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENIncorrect or inappropriate output or functionality 不正确或不适当的输出或功能性Yes NoDesign LabelIncorrect measurement不正确测量Yes NoDesign LabelErro
19、neous data transfer错误资料传递Yes NoDesign LabelLoss or deterioration of function功能丧失或恶化Yes NoDesign LabelUSE ERROR使用错误HAZARD APPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENAttentional failure保养不当Yes NoDesign LabelMemory failure记忆故障Yes NoDesign LabelRule-basedfail
20、ure设立基准规那么故障Yes NoDesign LabelKnowledge- based failure系统故障Yes NoDesign LabelRoutine Violation违反常规Yes NoDesign LabelTABLE-E.1 INFOMATION HAZARDS信息危害LABELING标签HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENIncomplete instructions for use不完全按照使用说明书操作Yes No
21、Design LabelInadequate description of performance characteristics不完整的工作特性说明书Yes NoDesign LabelInadequate specification of accessories to be used with the medical device用于医疗器械的附件说明书不完整Yes NoDesign LabelInadequate disclosure of limitations有限的改良但不完整Yes NoDesign LabelOPERATATING INSTRUCTIONS操作说明HAZARDAP
22、PLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENInadequate specification of accessories to be used with the medical device用于医疗器械的附件说明书不完整Yes NoDesign LabelInadequate specification of pre-use checks预先使用Yes NoDesign LabelOver-complicated operating instructions过于复杂
23、的操作说明书Yes NoDesign LabelWARNINGS警告HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENWarnings of side effects副作用的警告Yes NoDesign LabelWarnings of hazards likely with re-use of single-use medical devices不安全警示可能与单纯重复使用医疗设备有关Yes NoDesign LabelSPECIFICATION OF S
24、ERVICE AND MAINTENANCE维护保养说明HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENInadequate specification of when service and maintenance is required当需要维护保养时,说明书不详尽Yes NoDesign LabelTABLE-E.2 INITIATING EVENTS AND CIRCUMSTANCES启动结果INCOMPLETE REQUIREMENTS, Ina
25、dequate Specification of:不完全要求,不充分的说明:HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENDesign parameters设计参数Yes NoDesign LabelOperating parameters操作参数Yes NoDesign LabelPerformance requirements性能要求Yes NoDesign LabelIn-service requirements (e.g. maintenance
26、, reprocessing)运行中要求维护,重复处理Yes NoDesign LabelEnd of life保质期完毕Yes NoDesign LabelMANUFACTURING PROCESSES, Insufficient control of:产品处理程序,没有完全控制:HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENChanges to manufacturing process改变产品的程序Yes NoDesign LabelMateria
27、ls / materials compatibility information材料兼容性报告Yes NoDesign LabelManufacturing processes生产程序Yes NoDesign LabelSubcontractors次承包商Yes NoDesign LabelTRANSPORT AND STORAGE运输和储存HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENInadequate packaging不适当包装Yes NoDes
28、ign LabelContamination or deterioration污染或变质Yes NoDesign LabelInappropriate environmental conditions不良的环境条件Yes NoDesign LabelENVIROMANTAL FACTORS环境因素HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENPhysical (e.g. heat, pressure, time)物理上的热,压力,时间Yes NoDesi
29、gn LabelChemical (e.g. cocorrosions, degradation, contamination)化学上的腐蚀,变质,污染Yes NoDesign LabelElectromagnetic fields (e.g. susceptibility to electromagnetic disturbance)电磁场易受电磁干扰Yes NoDesign LabelInadequate supply of power能量不能充分供给Yes NoDesign LabelInadequate supply of coolant冷却剂不能充分供给Yes NoDesign La
30、belCLEANING, DISENFECTING, AND STERIALIZATIONHAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENLack of, or inadequate specification for, validated procedures for cleaning, disinfecting and sterilization.缺乏或对有效性没有充分的说明,对清洁消毒杀菌过程没有充分说明Yes NoDesign LabelInade
31、quate conduct for cleaning, disinfecting and sterilization没有对清洁消毒杀菌进展充分的指导Yes NoDesign LabelDISPOSAL AND SCRAPPING处理和报废HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENNo, or inadequate information provided.提供不准确的信息Yes NoDesign LabelUse error使用误差Yes NoDes
32、ign LabelFORMULATION明确地表达HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENBiodegrationYes NoDesign LabelBiocompatibility生物适应性Yes NoDesign LabelNo Information or inadequate specification provided没有提供信息或充分说明Yes NoDesign LabelInadequate warnings of hazards a
33、ssociated with incorrect formulations不充分的不安全警告与不正确的表达Yes NoDesign LabelUse error使用误差Yes NoDesign LabelHUMAN FACTORS, Potential for use errors triggered by design flaws, such as:人为因素,设计缺陷而引起可能使用误差HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFECTS OF FAILURERESPONSERISKINDEXACTION TAKENConfusing o
34、r missing instructions for use使用说明书使用不当或丧失Yes NoDesign LabelComplex or confusing control system复杂或混乱的控制系统Yes NoDesign LabelAmbiguous or unclear device state含混不清的设计说明Yes NoDesign LabelAmbiguous or unclear presentation of settings, measurements or other information设置说明,尺寸或其它介绍含混不清Yes NoDesign LabelMis
35、representation of results结果误报Yes NoDesign LabelInsufficient visibility, audibility of tactility触感的可见度,可听度不够Yes NoDesign LabelPoor mapping of controls to actions, or of displayed information to actual state对具体的情态信息显示,对动作的控制影象都很差Yes NoDesign LabelControversial modes or mapping as compared to existing
36、equipment与现有的装置相比,模式和影象都有争议Yes NoDesign LabelUse by unskilled/untrained personnel设备由不熟练和没有培训过的人员来操控Yes NoDesign LabelInsufficient warnings of side effects副作用的警告不够完备Yes NoDesign LabelInadequate warnings of hazards associated with re-use of single-use medical devices不适当的警告拌有单一反复使用医疗设备 Yes No Design La
37、belIncorrect measurement and other metrological aspects. 不正确的尺寸和其它计量方法Yes NoDesign LabelIncompatibility with consumables/accessories/other medical devices与耗材,附件或其它医疗设备不相容Yes NoDesign LabelSlip, laps and mistakes错误Yes NoDesign LabelFAILURE MODES错误模式HAZARDAPPLICABLEPOTENTIALFAILURECAUSE OFFAILUREEFFEC
38、TS OF FAILURERESPONSERISKINDEXACTION TAKENUnexpected loss of electrical/mechanical integrity电气机械完善的不可预测的损耗Yes NoDesign LabelDeterioration in function (e.g. gradual occlusion of fluid/gas path, or change in resistanceto flow, electrical conductivity) as a result of ageing, wear and repeated use功能退化液体
39、气路逐渐堵塞或在抵抗流量电导率方面的改变Yes NoDesign LabelFatigue failure疲劳破损Yes NoDesign LabelInadequate warnings of hazards associated with incorrect formulations不充分的不安全警示并拌有不正确的说明Yes NoDesign LabelUse error使用失误Yes NoDesign LabelConclusion:It has been concluded through the process of risk analysis that this is a low
40、risk device and any risks that existed were eliminated or reduced through safety testing, proper choice of materials, sterilization validation, and thorough instructions for use. The risk analysis was evaluated in regarding the European Medical Device Directives (MDD), Annex 1, Chapter 1, point 1 and 2.结论:通过风险的过程分析我们从中可以得出结论,这是一个低风险的设备经过安全测试选择适宜的材料,有效消毒,认真阅读使用说明书,何存在的风险都是可以排除的或者说是可以减少的依照有关的欧洲医疗设备法令第一章,第一和二节的附件一可以评估分析风险
- 温馨提示:
1: 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
2: 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
3.本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
5. 装配图网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
